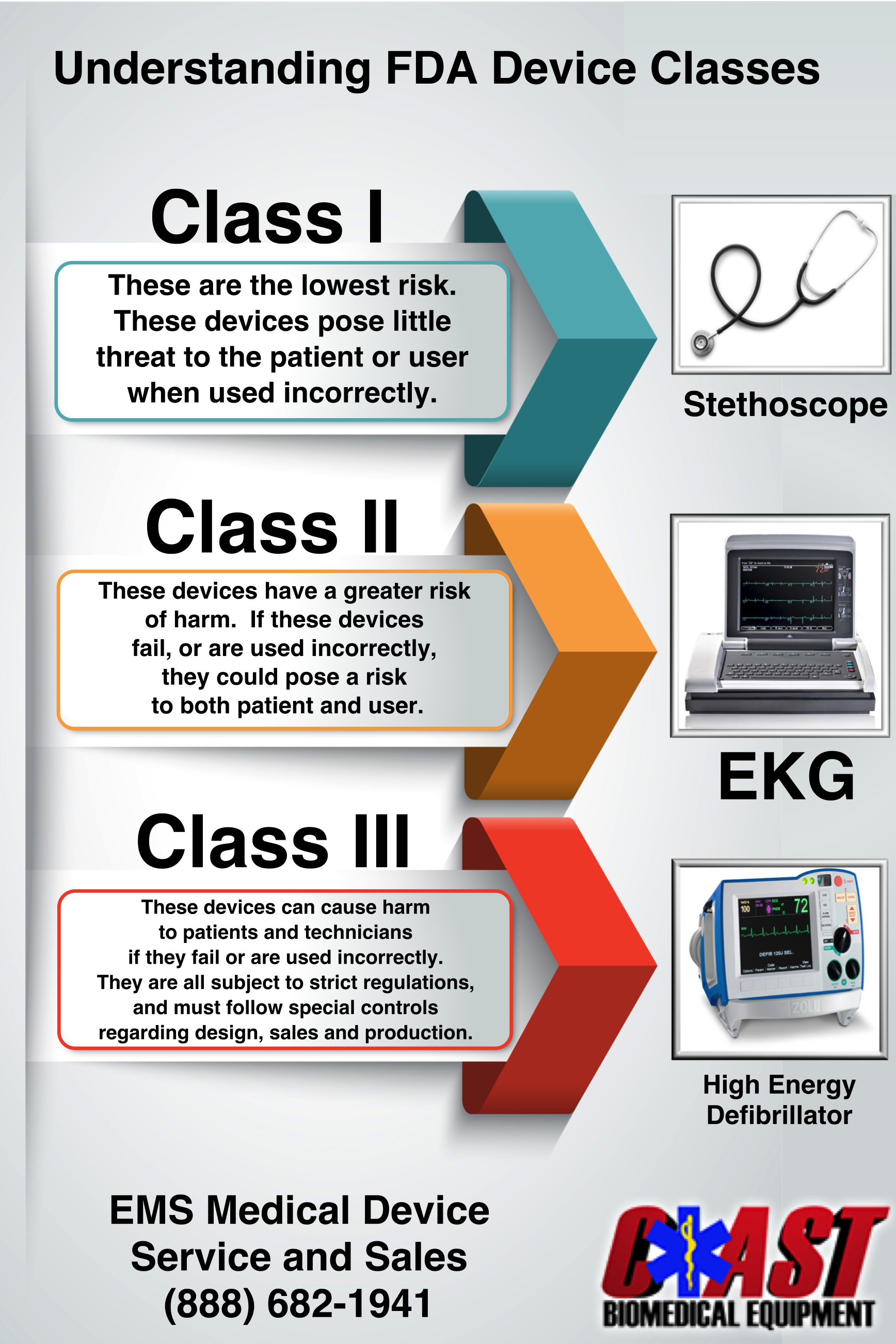
Fda Medical Device Prices . voluntary halal certification is scheduled to begin in 2021 but certification will not be mandatory for medical devices. premarket approvals (pma) database. when submitting a 510 (k) you must compare your device to one or more legally marketed devices and then make. Pay the relevant fee for 510 (k) application review and submit your 510 (k) documentation to the. Fda regulates the sale of medical device products in the. the food and drug administration (fda) is announcing the fee rates and payment procedures for medical. the food and drug administration (fda) is announcing the fee rates and payment procedures for medical.
from coastbiomed.com
the food and drug administration (fda) is announcing the fee rates and payment procedures for medical. the food and drug administration (fda) is announcing the fee rates and payment procedures for medical. voluntary halal certification is scheduled to begin in 2021 but certification will not be mandatory for medical devices. Fda regulates the sale of medical device products in the. premarket approvals (pma) database. Pay the relevant fee for 510 (k) application review and submit your 510 (k) documentation to the. when submitting a 510 (k) you must compare your device to one or more legally marketed devices and then make.
Blog Coast Biomedical Equipment
Fda Medical Device Prices when submitting a 510 (k) you must compare your device to one or more legally marketed devices and then make. the food and drug administration (fda) is announcing the fee rates and payment procedures for medical. the food and drug administration (fda) is announcing the fee rates and payment procedures for medical. Pay the relevant fee for 510 (k) application review and submit your 510 (k) documentation to the. voluntary halal certification is scheduled to begin in 2021 but certification will not be mandatory for medical devices. when submitting a 510 (k) you must compare your device to one or more legally marketed devices and then make. premarket approvals (pma) database. Fda regulates the sale of medical device products in the.
From www.greenlight.guru
What is the FDA Medical Device Registration Process? Fda Medical Device Prices voluntary halal certification is scheduled to begin in 2021 but certification will not be mandatory for medical devices. the food and drug administration (fda) is announcing the fee rates and payment procedures for medical. the food and drug administration (fda) is announcing the fee rates and payment procedures for medical. premarket approvals (pma) database. when. Fda Medical Device Prices.
From synectic.net
Medical Device FDA Regulations Infographic Synectic Fda Medical Device Prices Fda regulates the sale of medical device products in the. when submitting a 510 (k) you must compare your device to one or more legally marketed devices and then make. Pay the relevant fee for 510 (k) application review and submit your 510 (k) documentation to the. premarket approvals (pma) database. the food and drug administration (fda). Fda Medical Device Prices.
From 510kfdapro.com
Que es el 510k Compliance 4 Devices Fda Medical Device Prices when submitting a 510 (k) you must compare your device to one or more legally marketed devices and then make. Pay the relevant fee for 510 (k) application review and submit your 510 (k) documentation to the. the food and drug administration (fda) is announcing the fee rates and payment procedures for medical. voluntary halal certification is. Fda Medical Device Prices.
From www.complianceg.com
FDA Medical Device 5 Stage Goals to Launch Medical Devices to Market Fda Medical Device Prices Fda regulates the sale of medical device products in the. voluntary halal certification is scheduled to begin in 2021 but certification will not be mandatory for medical devices. when submitting a 510 (k) you must compare your device to one or more legally marketed devices and then make. the food and drug administration (fda) is announcing the. Fda Medical Device Prices.
From angelanjohnson.com
Medical Devices Angela N Johnson Fda Medical Device Prices premarket approvals (pma) database. Fda regulates the sale of medical device products in the. the food and drug administration (fda) is announcing the fee rates and payment procedures for medical. voluntary halal certification is scheduled to begin in 2021 but certification will not be mandatory for medical devices. when submitting a 510 (k) you must compare. Fda Medical Device Prices.
From customscity.com
FDA Medical Device (Unique Device Identifier Requirements) Fda Medical Device Prices Pay the relevant fee for 510 (k) application review and submit your 510 (k) documentation to the. voluntary halal certification is scheduled to begin in 2021 but certification will not be mandatory for medical devices. the food and drug administration (fda) is announcing the fee rates and payment procedures for medical. the food and drug administration (fda). Fda Medical Device Prices.
From meddevice.blogspot.com
Medical Device Blog 510(k) Infographic Fda Medical Device Prices the food and drug administration (fda) is announcing the fee rates and payment procedures for medical. voluntary halal certification is scheduled to begin in 2021 but certification will not be mandatory for medical devices. the food and drug administration (fda) is announcing the fee rates and payment procedures for medical. when submitting a 510 (k) you. Fda Medical Device Prices.
From www.nsmedicaldevices.com
Seven major medical devices approved by the US FDA in 2020 Fda Medical Device Prices premarket approvals (pma) database. the food and drug administration (fda) is announcing the fee rates and payment procedures for medical. Fda regulates the sale of medical device products in the. Pay the relevant fee for 510 (k) application review and submit your 510 (k) documentation to the. the food and drug administration (fda) is announcing the fee. Fda Medical Device Prices.
From www.modernhealthcare.com
FDA offers guide on wireless healthcare devices Modern Healthcare Fda Medical Device Prices the food and drug administration (fda) is announcing the fee rates and payment procedures for medical. Fda regulates the sale of medical device products in the. voluntary halal certification is scheduled to begin in 2021 but certification will not be mandatory for medical devices. the food and drug administration (fda) is announcing the fee rates and payment. Fda Medical Device Prices.
From coastbiomed.com
Blog Coast Biomedical Equipment Fda Medical Device Prices Fda regulates the sale of medical device products in the. when submitting a 510 (k) you must compare your device to one or more legally marketed devices and then make. the food and drug administration (fda) is announcing the fee rates and payment procedures for medical. voluntary halal certification is scheduled to begin in 2021 but certification. Fda Medical Device Prices.
From premier-research.com
Medical Device Trials What You Need to Know About U.S. Regulations Fda Medical Device Prices voluntary halal certification is scheduled to begin in 2021 but certification will not be mandatory for medical devices. Fda regulates the sale of medical device products in the. Pay the relevant fee for 510 (k) application review and submit your 510 (k) documentation to the. when submitting a 510 (k) you must compare your device to one or. Fda Medical Device Prices.
From www.provisionfda.com
FDA Medical Device User Fees Increase 7 for 2021 Fiscal Year Fda Medical Device Prices Pay the relevant fee for 510 (k) application review and submit your 510 (k) documentation to the. the food and drug administration (fda) is announcing the fee rates and payment procedures for medical. Fda regulates the sale of medical device products in the. the food and drug administration (fda) is announcing the fee rates and payment procedures for. Fda Medical Device Prices.
From www.greenlight.guru
Understanding the FDA Medical Device Classification System Fda Medical Device Prices Fda regulates the sale of medical device products in the. the food and drug administration (fda) is announcing the fee rates and payment procedures for medical. Pay the relevant fee for 510 (k) application review and submit your 510 (k) documentation to the. when submitting a 510 (k) you must compare your device to one or more legally. Fda Medical Device Prices.
From rockhealth.com
Pulse check An analysis of the FDA’s list of AI and MLenabled Fda Medical Device Prices Fda regulates the sale of medical device products in the. voluntary halal certification is scheduled to begin in 2021 but certification will not be mandatory for medical devices. the food and drug administration (fda) is announcing the fee rates and payment procedures for medical. Pay the relevant fee for 510 (k) application review and submit your 510 (k). Fda Medical Device Prices.
From www.rimsys.io
FDA listed, cleared, approved, granted what do these mean, and what’s Fda Medical Device Prices Fda regulates the sale of medical device products in the. premarket approvals (pma) database. the food and drug administration (fda) is announcing the fee rates and payment procedures for medical. when submitting a 510 (k) you must compare your device to one or more legally marketed devices and then make. the food and drug administration (fda). Fda Medical Device Prices.
From www.medicalpriceonline.com
FDA Approval Process for Medical Devices Medical Price Online Fda Medical Device Prices Pay the relevant fee for 510 (k) application review and submit your 510 (k) documentation to the. premarket approvals (pma) database. the food and drug administration (fda) is announcing the fee rates and payment procedures for medical. when submitting a 510 (k) you must compare your device to one or more legally marketed devices and then make.. Fda Medical Device Prices.
From angelanjohnson.com
DrugDevice Combination Treatments and Diagnostics Roundtable Angela Fda Medical Device Prices the food and drug administration (fda) is announcing the fee rates and payment procedures for medical. when submitting a 510 (k) you must compare your device to one or more legally marketed devices and then make. Pay the relevant fee for 510 (k) application review and submit your 510 (k) documentation to the. voluntary halal certification is. Fda Medical Device Prices.
From www.slideshare.net
Understanding FDA Requirements Medical Devices Fda Medical Device Prices voluntary halal certification is scheduled to begin in 2021 but certification will not be mandatory for medical devices. Pay the relevant fee for 510 (k) application review and submit your 510 (k) documentation to the. premarket approvals (pma) database. Fda regulates the sale of medical device products in the. the food and drug administration (fda) is announcing. Fda Medical Device Prices.